IndiaMART, a leading online marketplace connecting buyers and suppliers, is becoming increasingly controversial for its role in facilitating the sale of pharmaceuticals. While ostensibly a B2B platform, IndiaMART’s practices have significant implications for global healthcare and safety, particularly in the U.S. This article examines how IndiaMART’s lack of stringent verification processes and focus on bulk sales contribute to the distribution of tainted medications and undermine the credibility of legitimate Indian pharmacies.
IndiaMART’s Verification Lapses
Questionable Verification Standards
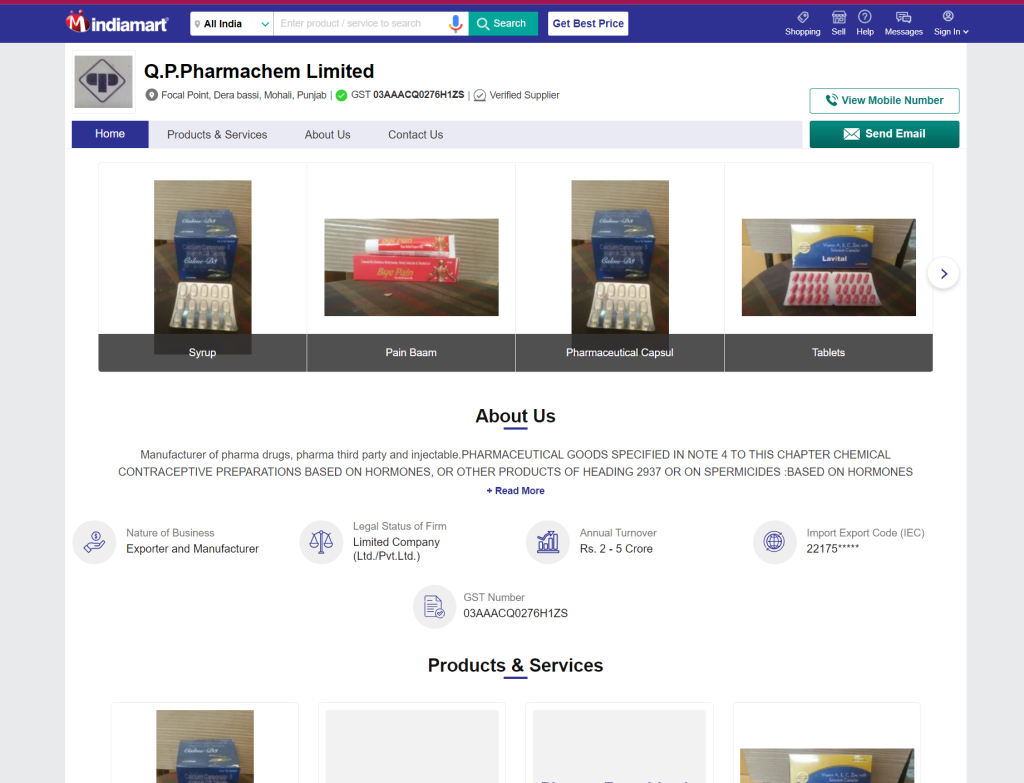
IndiaMART claims to verify suppliers, yet incidents like the one involving QP Pharmachem reveal serious flaws. Despite producing tainted medications that led to a World Health Organization (WHO) alert and losing their license, QP Pharmachem remained a verified supplier on IndiaMART. This lack of rigorous verification allows dubious firms to sell products, including veterinary medicines and anabolic steroids, without adequate oversight.
No Real Inspections
Suppliers on IndiaMART are not subjected to thorough inspections. The platform’s verification process is superficial, primarily focused on the submission of minimal documentation, with no robust checks to ensure quality or compliance with ethical standards. This creates a marketplace where substandard products can easily proliferate.
IndiaMART even ranks QP Pharmachem is a Verified Supplier, despite the company being implicated in producing tainted medications that triggered a World Health Organization (WHO) alert and losing their license. This raises serious questions about IndiaMART’s verification process and its impact on global health.

Bulk Sales and Legal Implications
B2B or B2C?
Though IndiaMART is marketed as a B2B platform, it facilitates direct-to-consumer (B2C) sales in bulk, which violates many countries’ laws regarding the importation of pharmaceuticals. For instance, U.S. regulations typically allow only a 90-day supply of medications for personal use. IndiaMART’s bulk sales encourage illegal importation practices, undermining regulatory frameworks designed to protect public health.
Illegal Resale
The focus on bulk sales enables individuals without proper licenses to import large quantities of drugs for illegal resale. This practice not only violates laws but also poses significant health risks, as these unregulated products may be unsafe or ineffective.
Consequences for Legitimate Pharmacies
Erosion of Trust
The unscrupulous practices on IndiaMART tarnish the reputation of all Indian pharmaceutical suppliers. Legitimate online pharmacies, such as those verified by Ashvin Medicara, are unfairly painted with the same brush. The perception that all Indian pharmaceutical products are substandard or unsafe is a significant barrier to access for consumers who rely on affordable medications.
Increased Scrutiny
U.S. Customs and Border Protection (CBP) and the FDA spend considerable resources inspecting packages from India due to the high volume of dubious products originating from platforms like IndiaMART. This increased scrutiny delays the delivery of legitimate 90-day supplies of personal medications, causing unnecessary hardships for patients.

Impact of Shadow Regulation
Influence of LegitScript and Others
Organizations like LegitScript, influenced by Big Pharma, use the activities on platforms like IndiaMART to justify their stringent criteria and the blocking of legitimate online pharmacies. These pseudo-regulatory bodies claim to protect consumers, but their actions often protect corporate interests by maintaining high drug prices and limiting access to affordable generics.
Payment Processor Restrictions
Visa, MasterCard, PayPal, and Venmo, guided by LegitScript’s certifications, refuse to process payments for pharmaceutical transactions involving Indian suppliers. This blockade reinforces the narrative that online Indian pharmacies are unsafe, pushing consumers towards higher-priced domestic options.
Call for Action
Government Intervention
To maintain the integrity of the Indian pharmaceutical industry and protect global health, the Indian government must regulate platforms like IndiaMART more stringently. This includes enforcing verification of both buyers and sellers and limiting sales to the legal 90-day supply for personal use.
Consumer Awareness
Consumers must be educated on the risks associated with purchasing medications from unverified sources. Promoting awareness about legitimate, verified online pharmacies can help ensure that individuals have access to safe and affordable medications.
Conclusion
IndiaMART’s business practices have significant implications for global health and safety. By allowing the sale of bulk medications to unverified buyers, the platform contributes to the spread of substandard and potentially dangerous pharmaceuticals. This not only endangers public health but also undermines the credibility of legitimate Indian pharmacies. It is imperative that regulatory bodies, both in India and internationally, take action to address these issues, ensuring that only verified suppliers and buyers can participate in the marketplace. By doing so, we can protect consumers and support the integrity of the pharmaceutical industry.
References:
- “Pharmaceutical Industry Lobbying.” OpenSecrets. Retrieved from OpenSecrets.org.
- “FDA User Fees and Pharmaceutical Influence.” PubMed. Retrieved from PubMed.
- “LegitScript and Its Role in Online Pharmacy Regulation.” PharmacyChecker Blog. Retrieved from PharmacyCheckerBlog.com.
- “IndiaMART and Pharmaceutical Sales.” Business Standard. Retrieved from Business-Standard.com.
- “World Health Organization Alerts.” WHO. Retrieved from WHO.int.
- “Customs and Border Protection Drug Inspection.” U.S. Customs and Border Protection. Retrieved from CBP.gov.
- “Impact of Payment Processor Restrictions on Online Pharmacies.” Journal of Medical Internet Research. Retrieved from JMIR.org.
- “Google and Microsoft Ad Policies.” Google Ads Policies. Retrieved from Google.com.
- “ICANN and Domain Seizures.” Internet Corporation for Assigned Names and Numbers. Retrieved from ICANN.org.
- “The Role of Payment Processors in Limiting Access to Pharmaceuticals.” Financial Times. Retrieved from FT.com.